Does it Fluoresce? – Scientific/first-principles approach
Posted On: Wednesday, April 1, 2015
Scientific/first-principles case study – a fluorescent mineral
In a separate post we talked about two approaches to addressing the question ‘Does it fluoresce?’ – empirical and scientific/first-principles. In this post we give an example of a scientific/first-principles solution of a fluorescence challenge. Switch to the empirical post.
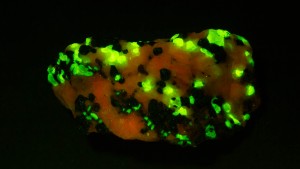
NIGHTSEA became involved in a fun project investigating mineral fluorescence under blue light excitation. One of the minerals we looked at was willemite, which was known to fluoresce green under both shortwave and longwave ultraviolet light. We found that it also fluoresces when excited by blue light, as seen in the photo here. (The red fluorescence is from calcite.)
- Calcite and willemite, white light
- Mineral fluorescence, calcite and willemite
The shortwave ultraviolet lights used by rockhounds emit primarily at 254 nm and the longwave lights at 365 nm, while the blue light we used peaks at about 445 nm. So empirically we knew that willemite fluoresced when excited at all these wavelengths. But that leaves questions unanswered. Are these wavelengths the ‘best’ in their respective spectral ranges? Are there other good wavelengths that we don’t know about?
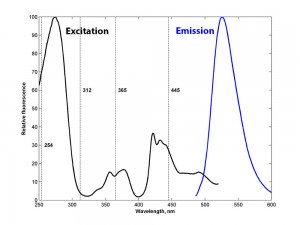
We wanted to explore this further and measured excitation and emission spectra of a willemite specimen using a laboratory spectrofluorometer, a FluoroMax-2 (SPEX Industries, now part of Horiba group). The figure below shows the normalized spectra. The emission spectrum was measured by setting the excitation at 450 nm and scanning the emission monochromator over a broad spectral range. Conversely, the excitation spectrum was measured by setting the emission monochromator to monitor fluorescence at 540 nm and scanning the excitation monochromator. The vertical lines in the figure are at 254, 312, 365, and 445 nm (312 nm is the wavelength peak of the medium wave ultraviolet lights used by mineralogists).
How does knowing the full excitation and emission spectra help?
For the shortwave ultraviolet range the data show that excitation at about 270 nm would produce about 50% brighter fluorescence than a 254 nm source of the same intensity. While this is true and perhaps interesting, this information is not particularly practical for general exploration since there are no economical, commercially available light sources that have strong emission at this wavelength. The ultraviolet wavelengths that are commonly used were not chosen based on science, but on engineering. Most commercial ultraviolet lights are based on mercury vapor discharge technology, and mercury vapor has specific atomic emission lines at the wavelengths already mentioned – 254, 312, and 365 nm. That enabled the manufacture of lamps for use both in the laboratory and the field.
If you have a new or critical application, however, knowing the full spectral properties as determined by the scientific/first-principles approach can be a tremendous help in selecting the light sources and filters you need to get the job done.
For more information or to get answers to your fluorescence questions contact NIGHTSEA.