Origin of the Model SFA Stereo Microscope Fluorescence Adapter
As is true for many scientific tools, the NIGHTSEA Model SFA Stereo Microscope Fluorescence Adapter was not developed as a ‘product’. Rather, it – or at least its precursor – was developed to meet a need in a specialized area of research. It was only later that other applications came to light. This is a brief account of how it came to be.
Updates
- NIGHTSEA’s development and commercialization of the SFA and related products for fluorescence has been recognized as an SBIR Success Story.
- In February 2021 NIGHTSEA founder Dr. Charles Mazel made a presentation to the NOAA Central Library about the original SBIR project and how it led to the current versions of the company’s products. Watch it here!
My (NIGHTSEA founder Charles Mazel) background was in exploring fluorescence in the underwater world. What had started out as personal curiosity had turned into a research career after I came back from my first Caribbean trip with images of fluorescing corals and only then learned how little was known about the phenomenon. With one thing leading to another I ended up with a PhD in Marine Biology and a position as a Principal Research Scientist at Physical Sciences Inc., pursuing funding opportunities for research on optical properties of marine organisms, with an emphasis on fluorescence.
In 2003 I responded to a topic in a NOAA (National Oceanic and Atmospheric Administration) SBIR solicitation. SBIR (Small Business Innovation Research) is a US government program that allocates a percentage of research funds to small businesses. The concept behind the program is that often there are small companies with innovative ideas but a shortage of cash. A modest grant can go a long way to jump start the early stages of proof of concept and prototyping. Agencies (NOAA, NASA, Department of Defense, NIH, etc.) issue periodic solicitations inviting proposals to address specific challenges. Phase I awards are for 6 months and are intended to demonstrate feasibility. If the results are promising you are invited to a write a proposal for a Phase II award that provides a larger sum of money and a 2 year period of performance, with the intent of bringing the idea from concept to advanced prototype development.
The title of the solicitation topic was ‘Fluorescence Imagery for Rapid Estimates of the Distribution and Abundance of Coral Recruits.’ Researchers needed better ways to find coral recruits – newly settled baby corals – in natural reef habitats. Coral larvae are either fertilized within the body of a polyp or in the water, through a process called spawning. In either case the larvae end up in the water , where they can drift for days before settling on the reef, hopefully to grow asexually into adults and continue to build healthy reef systems. (The process of coral reproduction, especially the mass spawning events, is one of the marvels of nature. For more information, check the links at the end of the article.)
Coral reef biologists have lots of questions about this process: What kinds of surfaces do the larvae prefer to settle on? What is the mortality rate after settlement? Does mortality vary by species? How rapidly does the coral grow in its early stages? What is the population structure of a reef in terms of coral age and size down to the recruit level? and more.
A coral reef is a very complex three-dimensional environment and newly settled coral larvae are only about a millimeter in diameter. This made it essentially impossible to locate juvenile corals until they were on the order of a centimeter or more, and even that required very painstaking searches of reef surfaces. A 1 cm coral is on the order of six months to a year old, so a significant portion of the early life history was missed.
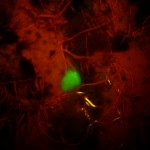
This is where fluorescence comes in. Many (perhaps most) corals fluoresce reasonably brightly. The benefit of fluorescence is that if something glows and the things around it don’t, or do but differently, it can make it much easier to see. Even a 1 mm coral can be easily seen in a casual search of the reef if you dive at night with the right lights, something I had already demonstrated through my early work on underwater fluorescence.
- Arrow pointing to juvenile coral, white light (c) Charles Mazel
- Juvenile coral <1mm diameter fluorescing (c) Charles Mazel
The NOAA topic was an obvious fit for my work so I wrote a proposal and was funded for Phase I. The results of that work were solid enough that I was also awarded Phase II funding. Several new technologies came out of that work, but in this article we are focused on the SFA.
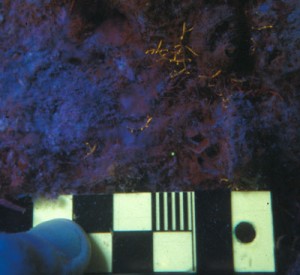
Because of the difficulty in locating baby corals on natural reef surfaces, marine biologists often resort to using artificial settlement tiles. These are essentially the rough sides of bathroom tiles, about 20 cm square. The tiles are placed on the reef at different locations, depths, and orientations to explore coral settlement preference. The researchers collect the tiles months later and examine them under a stereo microscope to locate and identify corals. The image pair below shows a complete tile under white light and also how it appears in fluorescence. You can see how the corals stand out.
- Settlement tile – white light (c) Charles Mazel
- Settlement tile – fluorescence. Scale in cm. (c) Charles Mazel
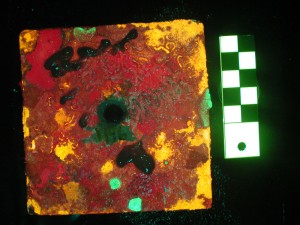
As part of the SBIR project we (myself and my science collaborator, Dr. Alina Szmant of UNC Wilmington) wanted to see if this benefit of fluorescence also extended to the microscope examination. The image pairs below show several small corals in white light and fluorescence. Under white light, in some cases the corals can be difficult to see because they blend with nearby features, and in others they might be hidden under a canopy of algae.
- Coral polyp on settlement tile – white light. (c) Alina Szmant
- Coral polyp on settlement tile – fluorescence. (c) Alina Szmant
- Coral polyp on settlement tile – white light. (c) Alina Szmant
- Coral polyp on settlement tile – fluorescence. (c) Alina Szmant
So the answer was clearly yes, fluorescence offered a valuable benefit. It was not a substitute for looking with white light since not all corals fluoresce strongly, but it definitely helped in finding more corals faster, and finding corals that could easily be missed under white light. This was one of those times when the answer is ‘Yes, but so what?’. We had clearly demonstrated the value of fluorescence, but fluorescence stereo microscopes were very expensive, too expensive for most marine biology budgets. Tropical marine stations are usually not well equipped with high end microscopes (not to mention the challenges of heat and humidity), and it is not ideal to ship an expensive and delicate high end microscope back and forth to remote tropical islands.
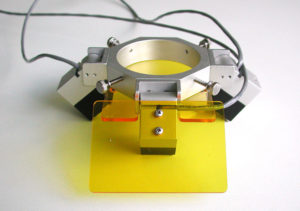
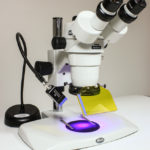
To address this challenge I developed a prototype system for mounting high intensity LEDs and a matching barrier filter on a conventional stereo microscope. This offered just one wavelength excitation/emission combination – blue light with a yellow longpass barrier filter – to replicate what we used for underwater searching. The pictures below show what this early system looked like. I made a few copies to demonstrate feasibility, and sold one to my science collaborator. At that time I did not appreciate the potential for a larger market and nothing more was done with it.
- Early prototype fluorescence adapter
- Early prototype fluorescence adapter, installed on microscope
Meanwhile NIGHTSEA existed as a sideline to my research work. It was originally established to provide fluorescence observation and photography equipment to marine scientists and sport divers. I had started selling a land version of the flashlights, using filter glasses instead of the filter visor used by divers, to science customers who wanted to sort GFP-transgenic organisms like mice. The fluorescent proteins in corals belong to the same family of proteins that were originally discovered in the jellyfish Aequorea aequorea, so the same technology that I was supplying to divers worked well in this application.
In 2010 I received a call from a researcher at Willamette College, a small teaching college in Oregon. He needed to sort GFP-transgenic fruit flies (Drosophila) for an experiment and had been about to spend ~$17k on a fluorescence stereo microscope. He felt like that was a lot for his limited application, looked around for alternatives, and found our BlueStar and filter glasses. He bought those and was able to sort his flies holding the light and wearing the glasses. He was very excited and told me that the fly community needed this solution. While the handheld approach worked for him it was obvious to me that it was not an ideal solution and I immediately thought of my old microscope adapter prototype. Motivated by the call I went back to the design, made some updates, and put together two hand-built prototypes. These were still single-wavelength systems, with blue excitation only. Armed only with these I booked an exhibit booth at the 2011 Drosophila Research Conference in San Diego. I had two core questions:
- Do you want this? – meaning, do you want a low-cost way to add fluorescence to a stereo microscope?
- Do you want this? – meaning, do you want the specific solution I have here.
The answer to the first question was a solid ‘Yes!’ and the answer to the second was ‘No – we need to work with more wavelengths’. While GFP had for years been the dominant fluorophore used for transgenics, by that time researchers were starting to use red fluorophores and needed to be able to work with multiple wavelength combinations.
I thanked the researchers for their enthusiastic interest and told them that I would be back the next year with a multi-wavelength system. There was one researcher in particular who seemed especially eager to acquire a system. She had funding for an experiment that would require sorting a very large number of flies. She had almost everything lined up but lacked the funds for the multiple fluorescence microscopes she needed to meet the workflow requirements. I finally asked her if she would be willing to use the single-wavelength system I was showing at the meeting, knowing that it would not be the final configuration, and proposed a price. She said she would take four. Once I committed to that I started asking others about interest in a purchase and by the time I left the show floor I had pre-sold seven. When I got home I sent an email out to everyone who had left their names at the booth saying that I was going to do a one-time build of that system, to see if there was additional interest. When the responses came in the pre-sale quantity came up to 22. This gave me some economy of scale. I made a run of 25 units and had no trouble selling the last three.
And after that it was off and running. There was definitely a need for the product and I started showing it to the other main model organism communities that worked with transgenics (zebrafish, C elegans, Xenopus) and going to conferences of groups like the Society for Developmental Biology. Over time the versatility of the system grew, adding additional wavelength combinations (we offer six now) and accessories like the Eclipse MicroTent, which can provide darkness if your microscope is not in a dark room and your fluorescence is not sufficiently bright. Besides aiding in research workflow, the system is popular for outfitting undergraduate teaching laboratories (because of its low cost), and for outreach activities in the community (because of its simplicity and portability). We have also branched out to other user communities and are solving problems for industry users for applications like failure analysis, for forensic scientists, and more.
It is gratifying that a solution that grew out of my own specialized work in underwater fluorescence and coral research has evolved into a useful and versatile tool now used in hundreds of laboratories and other locations worldwide.
Links